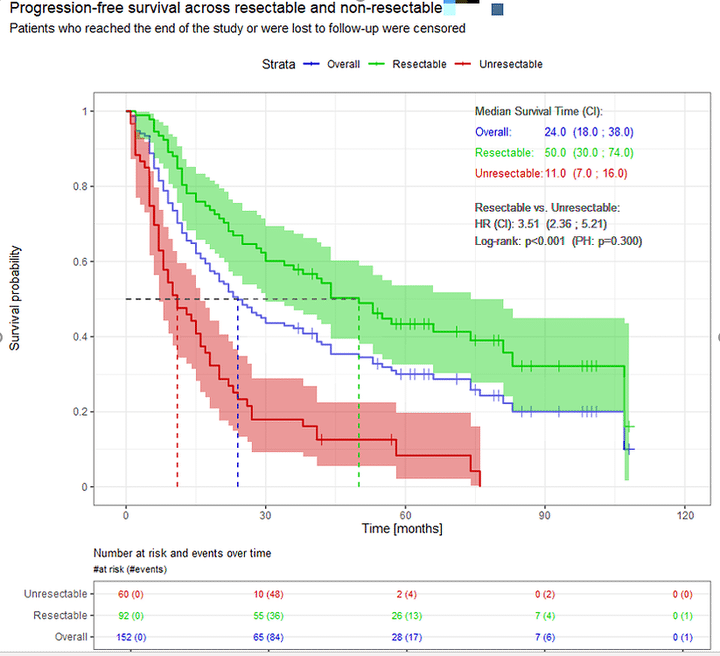
Survival Analysis : Part 2
doing survival analysis in R
functions
The core functions we’ll use out of the survival package include:
Surv(): Creates a survival object.survfit(): Fits a survival curve using either a formula, of from a previously fitted Cox model.coxph(): Fits a Cox proportional hazards regression model.
Other optional functions you might use include:
cox.zph(): Tests the proportional hazards assumption of a Cox regression model.survdiff(): Tests for differences in survival between two groups using a log-rank / Mantel-Haenszel test.[^1]
Surv() creates the response variable, and typical usage takes the time to event,[^time2] and whether or not the event occured (i.e., death vs censored). survfit() creates a survival curve that you could then display or plot. coxph() implements the regression analysis, and models specified the same way as in regular linear models, but using the coxph() function.
Data description
- status : event at discharge (alive or dead)
- sex : male or female
- dm : diabetes (yes or no)
- gcs : Glasgow Coma Scale (value from 3 to 15)
- sbp : Systolic blood pressure (mmHg)
- dbp : Diastolic blood pressure (mmHg)
- wbc : Total white cell count
- time2 : days in ward
- stroke_type : surv_data type (Ischaemic surv_data or Haemorrhagic surv_data)
- referral_from : patient was referred from a hospital or not from a hospital
setup
# load in packages
library(tidyverse)
library(rms)
library(broom)
library(survival)
library(survminer)
library(data.table)
library(DT)
surv_data <- read_csv('stroke_data.csv')
take a look at the dataset
kableExtra::kbl(head(surv_data))
| doa | dod | status | sex | dm | gcs | sbp | dbp | wbc | time2 | stroke_type | referral_from |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 17/2/2011 | 18/2/2011 | alive | male | no | 15 | 151 | 73 | 12.5 | 1 | IS | non-hospital |
| 20/3/2011 | 21/3/2011 | alive | male | no | 15 | 196 | 123 | 8.1 | 1 | IS | non-hospital |
| 9/4/2011 | 10/4/2011 | dead | female | no | 11 | 126 | 78 | 15.3 | 1 | HS | hospital |
| 12/4/2011 | 13/4/2011 | dead | male | no | 3 | 170 | 103 | 13.9 | 1 | IS | hospital |
| 12/4/2011 | 13/4/2011 | alive | female | yes | 15 | 103 | 62 | 14.7 | 1 | IS | non-hospital |
| 4/5/2011 | 5/5/2011 | dead | female | no | 3 | 91 | 55 | 14.2 | 1 | HS | hospital |
Creating a Survival Object
The survival object is created by the function survival::Surv, which typically requires two arguments: event and time. The survival object will be used as the outcome by the survival analysis methods we explore.
Some key components of this survfit object that will be used to create survival curves include:
time: the timepoints at which the curve has a step, i.e. at least one event occurredsurv: the estimate of survival at the correspondingtime
surv_object <- survival::Surv(
event = surv_data$status=="dead",
time = surv_data$time2
)
head(surv_object, 20)
#> [1] 1+ 1+ 1 1 1+ 1 1+ 1+ 1 1+ 1+ 1+ 1 1 1+ 1 1+ 1 1+ 1+
Estimating the Survival Function: Kaplan-Meier Estimator
The Kaplan-Meier (KM) Estimator is a non-parametric method that estimates the survival probabilities at each time an event occurs. We will use the function survival::survfit(), which uses the KM Estimator, to estimate survival probabilities from our data.
survfit requires two arguments:
- A formula object where the outcome is a survival object
- A data frame
km_result <- survival::survfit(
surv_object ~ 1,
data = surv_data
)
The Output of survfit
#> # A tibble: 17 x 5
#> TIME N_RISK N_EVENT CENSOR SURVIVAL
#> <dbl> <dbl> <dbl> <dbl> <dbl>
#> 1 1 213 9 14 0.958
#> 2 2 190 4 20 0.938
#> 3 3 166 4 32 0.915
#> 4 4 130 4 36 0.887
#> 5 5 90 5 20 0.838
#> 6 6 65 3 6 0.799
#> 7 7 56 4 6 0.742
#> 8 9 42 1 8 0.724
#> 9 10 37 1 1 0.705
#> 10 12 33 4 5 0.619
#> 11 14 24 2 2 0.568
#> 12 18 19 1 3 0.538
#> 13 22 15 1 5 0.502
#> 14 25 9 2 4 0.390
#> 15 28 5 1 1 0.312
#> 16 29 4 1 0 0.234
#> 17 41 2 1 1 0.117
The survival probabilities for all patients:
>KM <- survfit(Surv(time = time2,event = status == "dead" ) ~ 1,
data = surv_data)
>summary(KM)
Call: survfit(formula = Surv(time = time2, event = status == "dead") ~
1, data = surv_data)
time n.risk n.event survival std.err lower 95% CI upper 95% CI
1 213 9 0.958 0.0138 0.9311 0.985
2 190 4 0.938 0.0168 0.9053 0.971
3 166 4 0.915 0.0198 0.8770 0.955
4 130 4 0.887 0.0237 0.8416 0.934
5 90 5 0.838 0.0310 0.7790 0.901
6 65 3 0.799 0.0367 0.7301 0.874
7 56 4 0.742 0.0438 0.6608 0.833
9 42 1 0.724 0.0462 0.6391 0.821
10 37 1 0.705 0.0489 0.6150 0.807
12 33 4 0.619 0.0587 0.5142 0.746
14 24 2 0.568 0.0642 0.4548 0.708
18 19 1 0.538 0.0674 0.4206 0.687
22 15 1 0.502 0.0718 0.3792 0.664
25 9 2 0.390 0.0892 0.2494 0.611
28 5 1 0.312 0.0998 0.1669 0.584
29 4 1 0.234 0.1009 0.1007 0.545
41 2 1 0.117 0.0970 0.0231 0.593
Next, we will estimate the survival probabilities for stroke type:
- this will give us two tables ,relating to two factors that we have in the data
>KM_str_type2 <- survfit(Surv(time = time2,
event = status == "dead" ) ~ stroke_type,
data = surv_data)
>summary(KM_str_type2)
Call: survfit(formula = Surv(time = time2, event = status == "dead") ~
stroke_type, data = surv_data)
stroke_type=HS
time n.risk n.event survival std.err lower 95% CI upper 95% CI
1 69 6 0.913 0.0339 0.8489 0.982
2 61 1 0.898 0.0365 0.8293 0.973
3 58 4 0.836 0.0453 0.7520 0.930
4 52 2 0.804 0.0489 0.7136 0.906
5 47 4 0.736 0.0554 0.6346 0.853
6 38 2 0.697 0.0589 0.5905 0.822
7 34 2 0.656 0.0621 0.5447 0.790
9 30 1 0.634 0.0638 0.5205 0.772
10 27 1 0.611 0.0656 0.4945 0.754
12 24 2 0.560 0.0693 0.4390 0.713
14 19 1 0.530 0.0717 0.4068 0.691
18 15 1 0.495 0.0751 0.3675 0.666
22 11 1 0.450 0.0806 0.3166 0.639
25 6 2 0.300 0.1019 0.1541 0.584
29 2 1 0.150 0.1176 0.0322 0.698
- this is another output ,technically they should be together in one call
stroke_type=IS
time n.risk n.event survival std.err lower 95% CI upper 95% CI
1 144 3 0.979 0.0119 0.956 1.000
2 129 3 0.956 0.0174 0.923 0.991
4 78 2 0.932 0.0241 0.886 0.980
5 43 1 0.910 0.0318 0.850 0.975
6 27 1 0.876 0.0451 0.792 0.970
7 22 2 0.797 0.0676 0.675 0.941
12 9 2 0.620 0.1223 0.421 0.912
14 5 1 0.496 0.1479 0.276 0.890
28 3 1 0.331 0.1671 0.123 0.890
41 1 1 0.000 NaN NA NA
Plot the survival probability
The KM estimate provides the survival probabilities. We can plot these probabilities to look at the trend of survival over time. The plot provides
- survival probability on the \(y-axis\)
- time on the \(x-axis\)
Plotting the Survival Function
survminer::ggsurvplot(
km_result,
pval = TRUE,
conf.int = TRUE,
xlab = "Time",
ylab = "Probability of dying"
)

Using the KM Estimator to Plot Multiple Survival Functions
km_result_jobs <- survival::survfit(surv_object ~stroke_type, data = surv_data)
survminer::ggsurvplot(km_result_jobs, pval = TRUE, xlab = "Time", ylab = "Probability of dying")

Making it more fency
- i always love to make the graphs more fency
- i have used ggplot2 extended functionalities to make the plots pretty neat
library(paletteer)
p1<-ggsurvplot(KM_str_type2,
data = surv_data,
palette = paletteer_d("ggsci::light_blue_material")[seq(2,10,2)],
size = 1.2, conf.int = FALSE,
legend.labs = levels(surv_data$stroke_type),
legend.title = "",
ggtheme = theme_minimal() +
theme(plot.title = element_text(face = "bold")),
title = "Probability of dying",
xlab = "Time",
ylab = "Probability of dying",
legend = "bottom", censor = FALSE)
p1$plot +
scale_x_continuous(expand = c(0, 0), breaks = seq(1,43,1),
labels = seq(1,43,1),
limits = c(0, 820)) +
scale_y_continuous(expand = c(0, 0), breaks = seq(0,1,0.2),
labels = scales::percent_format(accuracy = 1)) +
theme_classic()

We can perform the Kaplan-Meier estimates for variable dm too:
KM_dm <- survfit(Surv(time = time2,
event = status == "dead" ) ~ dm,
data = surv_data)
summary(KM_dm)
#> Call: survfit(formula = Surv(time = time2, event = status == "dead") ~
#> dm, data = surv_data)
#>
#> dm=no
#> time n.risk n.event survival std.err lower 95% CI upper 95% CI
#> 1 141 8 0.943 0.0195 0.9058 0.982
#> 2 122 4 0.912 0.0242 0.8661 0.961
#> 3 102 2 0.894 0.0268 0.8434 0.949
#> 4 82 2 0.873 0.0303 0.8152 0.934
#> 5 54 5 0.792 0.0441 0.7100 0.883
#> 6 40 3 0.732 0.0524 0.6366 0.843
#> 7 34 2 0.689 0.0575 0.5854 0.812
#> 10 24 1 0.661 0.0619 0.5498 0.794
#> 12 20 4 0.529 0.0771 0.3971 0.703
#> 18 13 1 0.488 0.0812 0.3521 0.676
#> 22 9 1 0.434 0.0884 0.2908 0.647
#> 25 4 1 0.325 0.1149 0.1627 0.650
#> 29 3 1 0.217 0.1171 0.0752 0.625
#>
#> dm=yes
#> time n.risk n.event survival std.err lower 95% CI upper 95% CI
#> 1 72 1 0.986 0.0138 0.9594 1.000
#> 3 64 2 0.955 0.0253 0.9070 1.000
#> 4 48 2 0.915 0.0367 0.8463 0.990
#> 7 22 2 0.832 0.0653 0.7137 0.971
#> 9 15 1 0.777 0.0811 0.6330 0.953
#> 14 9 2 0.604 0.1248 0.4030 0.906
#> 25 5 1 0.483 0.1471 0.2662 0.878
#> 28 2 1 0.242 0.1860 0.0534 1.000
#> 41 1 1 0.000 NaN NA NA
And then we can plot the survival estimates for patients with and without diabetes:
p2<-ggsurvplot(KM_dm,
data = surv_data,
palette = paletteer_c("grDevices::Set 2", 12),
size = 1.2, conf.int = FALSE,
legend.labs = levels(surv_data$dm),
legend.title = "",
ggtheme = theme_minimal() +
theme(plot.title = element_text(face = "bold")),
title = "Probability of dying",
xlab = "Time till discharge",
ylab = "Probability of dying",
legend = "bottom", censor = FALSE)
p2

- Typically we will also want to see the numbers at risk in a table below
the x-axis. We can add this using
add_risktable():
library(ggsurvfit)
survfit2(Surv(time = time2,event = status == "dead" ) ~ dm, data = surv_data) %>%
ggsurvfit() +
labs(
x = "Days",
y = "Overall survival probability"
) +
add_confidence_interval() +
add_risktable()

Comparing Kaplan-Meier estimates across groups
There are a number of available tests to compare the survival estimates between groups based on KM. The tests include:
- log-rank (default)
- peto-peto test
Log-rank test
to answer question if the survival estimates are different between levels or groups we can use statistical tests for example the log rank and the peto-peto tests.
For all the test, the null hypothesis is that that the survival estimates between levels or groups are not different. For example, to do that:
>survdiff(Surv(time = time2,
event = status == "dead") ~ stroke_type,
data = surv_data,
rho = 0)
Call:
survdiff(formula = Surv(time = time2, event = status == "dead") ~
stroke_type, data = surv_data, rho = 0)
N Observed Expected (O-E)^2/E (O-E)^2/V
stroke_type=HS 69 31 24.2 1.92 4.51
stroke_type=IS 144 17 23.8 1.95 4.51
Chisq= 4.5 on 1 degrees of freedom, p= 0.03
And for the survival estimates based on diabetes status:
>survdiff(Surv(time = time2,
event = status == "dead") ~ dm,
data = surv_data,
rho = 0)
Call:
survdiff(formula = Surv(time = time2, event = status == "dead") ~
dm, data = surv_data, rho = 0)
N Observed Expected (O-E)^2/E (O-E)^2/V
dm=no 141 35 29.8 0.919 2.54
dm=yes 72 13 18.2 1.500 2.54
Chisq= 2.5 on 1 degrees of freedom, p= 0.1
The survival estimates between patients with and without diabetes (dm status yes vs no groups) are not different (p-value = 0.1).
Cox PH General model
The Cox model is expressed by the hazard function denoted by \(h(t)\). This model can be used to fit univariable and multivariable regression models that have survival outcomes. The hazard function can be interpreted as the risk of dying at time t. It can be estimated as follow:
\(h(t, X_{i}) = h_{0}(t)e^{ \sum_{j=1}^{p} \beta_{j}X_{i,j}} = h_{0}(t)exp(\beta_{1}X_{i,1} + ... +\ beta_{p}X_{i,p})\)
where:
- \(h(t)\) is the hazard, the instantaneous rate at which events occur.
- \(h_{0}(t)\) is called the baseline hazards (when all X’s are equal to 0), depends on \(t\)
- \(X = (X_{1}, X_{2},..., X_{p})\) explanatory/predictor variables
- \(e^{ \sum_{i=1}^{p} \beta_{i}X_{i}}\), depends only on X’s, called .
Because the baseline hazard \(h_{0}(t)\) is an unspecified function, the Cox model us a semiparametric model.
The Cox model can be written as a multiple linear regression of the logarithm of the hazard on the variables \(X_{i}\), with the baseline hazard, \(h_{0}(t)\), being an ‘intercept’ term that varies with time.
$$log(h(t, X_{i})) = log(h_{0}(t)) + \sum_{j=1}^{p} \beta_{j}X_{i,j}$$
We can compute the hazard ratio, which is the ratio of hazards between two groups at any particular point in time: “hazard for one individual divided by the hazard for a different individual”.
$$\hat{HR} = \frac{\hat{h}(t, X^{*})}{\hat{h}(t, X)} = e^{ \sum_{i=1}^{p} \beta_{i} (X^{*}_{i} - X_{i})}$$
with:
\(X^{*}\): set of predictors for one individual
X: set of predictors for the other individual
This model shows that the hazard ratio is equal to \(e^{ \sum_{i=1}^{p} \beta_{i} (X^{*}_{i} - X_{i})}\), and remains constant over time t (hence the name proportional hazards regression). In this sense, we do not need the baseline hazard because we can interpret coefficients as hazard ratios.
A hazard ratio above 1 indicates a covariate that is positively associated with the event probability, and thus negatively associated with the length of survival.
In summary,
- HR = 1 : No effect
- HR < 1: Reduction in the hazard
- HR > 1: Increase in Hazard
As a note, in clinical studies, a covariate with hazard ratio :
- greater than 1 (i.e.: b>0) is called bad prognostic factor.
- smaller than 1 (i.e.: b<0) is called good prognostic factor.
As a consequence, a major assumption of this model is that the HR is constant over time because it is independent of time. Or equivalently that the hazard for one individual is proportional to the hazard for any other individual, where the proportionality constant is independent of time.
It is possible, nevertheless, to consider X’s which do involve t. Such X’s are called time-dependent variables. If time-dependent variables are considered, the Cox model form may still be used, but such a model no longer satisfies the PH assumption, and is called the extended Cox model.
Compute the Cox Model
- The coxph() function uses the same syntax as lm(), glm(), etc. The response variable you create with Surv() goes on the left hand side of the formula, specified with a ~. Explanatory variables go on the right side.
COX PH model with surv_data type variable only
(surv_data_stype <- coxph(Surv(time2,status == 'dead') ~ stroke_type
,data = surv_data))
#> Call:
#> coxph(formula = Surv(time2, status == "dead") ~ stroke_type,
#> data = surv_data)
#>
#> coef exp(coef) se(coef) z p
#> stroke_typeIS -0.6622 0.5157 0.3172 -2.088 0.0368
#>
#> Likelihood ratio test=4.52 on 1 df, p=0.03344
#> n= 213, number of events= 48
interpretting
The effect of surv_data type is significantly related to survival (p-value = 0.0368), with better survival in Ischaemic surv_data in comparison to the other type (hazard ratio of dying = 0.5157).
The model is statistically significant. That 0.03344 p-value of the model is really close to the p = 0.03 p-value we saw on the Kaplan-Meier nodel as well as the likelihood ratio test = 4.52 is close to the log-rank chi-square (4.5) in the Kaplan-Meier model.
\(e^{\beta_{1}}\) = \(e^{-0.6622}\) = 0.5157 is the hazard ratio - the multiplicative effect of that variable on the hazard rate (for each unit increase in that variable). Ischaemic surv_data patients have 0.588 (~ 60%) times the hazard of dying in comparison to haemorage.
Model Building
this is important when perfoming statistical analysis
- first build a model with all varibles
>surv_data_stype <- coxph(Surv(time2,status == 'dead') ~ gcs + stroke_type
+ sex + dm + sbp ,data = surv_data)
>summary(surv_data_stype)
Call:
coxph(formula = Surv(time2, status == "dead") ~ gcs + stroke_type +
sex + dm + sbp, data = surv_data)
n= 213, number of events= 48
coef exp(coef) se(coef) z Pr(>|z|)
gcs -0.170038 0.843633 0.037167 -4.575 4.76e-06 ***
stroke_typeIS -0.103523 0.901655 0.346749 -0.299 0.765
sexmale -0.203488 0.815880 0.334159 -0.609 0.543
dmyes -0.439913 0.644093 0.343960 -1.279 0.201
sbp -0.001765 0.998237 0.004017 -0.439 0.660
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
exp(coef) exp(-coef) lower .95 upper .95
gcs 0.8436 1.185 0.7844 0.9074
stroke_typeIS 0.9017 1.109 0.4570 1.7791
sexmale 0.8159 1.226 0.4238 1.5706
dmyes 0.6441 1.553 0.3282 1.2639
sbp 0.9982 1.002 0.9904 1.0061
Concordance= 0.78 (se = 0.035 )
Likelihood ratio test= 28.88 on 5 df, p=2e-05
Wald test = 27.71 on 5 df, p=4e-05
Score (logrank) test = 31.45 on 5 df, p=8e-06
- the estimate which is the log hazard. If you exponentiate it, you will get hazard ratio
- the standard error
- the p-value
- the confidence intervals for the log hazard
stroke_type <- coxph(Surv(time2,status == 'dead') ~ gcs + stroke_type+dbp+wbc
+ sex + dm + sbp ,data = surv_data)
tidy(surv_data_stype,
exponentiate = TRUE)
#> # A tibble: 1 x 5
#> term estimate std.error statistic p.value
#> <chr> <dbl> <dbl> <dbl> <dbl>
#> 1 stroke_typeIS 0.516 0.317 -2.09 0.0368
using rms package
fatal_mv1<-rms::cph(Surv(time2,status == 'dead') ~ gcs + stroke_type+dbp+wbc
+ sex + dm + sbp ,data = surv_data)
variable importance
plot(anova(fatal_mv1),margin=c("chisq","d.f","P"))

now we create univariate models as in logistic regression
By using tbl_uvregression() we can generate simple univariable model for all covariates in one line of code. In return, we get the crude HR for all the covariates of interest.
library(gt)
library(gtsummary)
surv_data |>
dplyr::select(time2, status, sex, dm, gcs, sbp, dbp, wbc,
stroke_type) |>
tbl_uvregression(
method = coxph,
y = Surv(time2, event = status == 'dead'),
exponentiate = TRUE,
pvalue_fun = ~style_pvalue(.x, digits = 3)
) |>
as_gt()
<tr class="gt_col_headings">
<th class="gt_col_heading gt_columns_bottom_border gt_left" rowspan="1" colspan="1" scope="col" id="<strong>Characteristic</strong>"><strong>Characteristic</strong></th>
<th class="gt_col_heading gt_columns_bottom_border gt_center" rowspan="1" colspan="1" scope="col" id="<strong>N</strong>"><strong>N</strong></th>
<th class="gt_col_heading gt_columns_bottom_border gt_center" rowspan="1" colspan="1" scope="col" id="<strong>HR</strong><span class="gt_footnote_marks" style="white-space:nowrap;font-style:italic;font-weight:normal;"><sup>1</sup></span>"><strong>HR</strong><span class="gt_footnote_marks" style="white-space:nowrap;font-style:italic;font-weight:normal;"><sup>1</sup></span></th>
<th class="gt_col_heading gt_columns_bottom_border gt_center" rowspan="1" colspan="1" scope="col" id="<strong>95% CI</strong><span class="gt_footnote_marks" style="white-space:nowrap;font-style:italic;font-weight:normal;"><sup>1</sup></span>"><strong>95% CI</strong><span class="gt_footnote_marks" style="white-space:nowrap;font-style:italic;font-weight:normal;"><sup>1</sup></span></th>
<th class="gt_col_heading gt_columns_bottom_border gt_center" rowspan="1" colspan="1" scope="col" id="<strong>p-value</strong>"><strong>p-value</strong></th>
</tr>
| sex | 213 | |||
| female | — | — | ||
| male | 0.71 | 0.37, 1.36 | 0.299 | |
| dm | 213 | |||
| no | — | — | ||
| yes | 0.60 | 0.31, 1.13 | 0.112 | |
| gcs | 213 | 0.84 | 0.79, 0.90 | <0.001 |
| sbp | 213 | 1.00 | 0.99, 1.01 | 0.617 |
| dbp | 213 | 1.00 | 0.98, 1.01 | 0.772 |
| wbc | 213 | 1.04 | 0.97, 1.11 | 0.270 |
| stroke_type | 213 | |||
| HS | — | — | ||
| IS | 0.52 | 0.28, 0.96 | 0.037 | |
| 1 HR = Hazard Ratio, CI = Confidence Interval | ||||
- it is clear that
gcshas the least p-value hence can be included in the model first
surv_data_gcs <- coxph(Surv(time = time2,event = status == 'dead') ~ gcs,
data = surv_data)
summary(surv_data_gcs)
#> Call:
#> coxph(formula = Surv(time = time2, event = status == "dead") ~
#> gcs, data = surv_data)
#>
#> n= 213, number of events= 48
#>
#> coef exp(coef) se(coef) z Pr(>|z|)
#> gcs -0.17454 0.83984 0.03431 -5.087 3.63e-07 ***
#> ---
#> Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
#>
#> exp(coef) exp(-coef) lower .95 upper .95
#> gcs 0.8398 1.191 0.7852 0.8983
#>
#> Concordance= 0.763 (se = 0.039 )
#> Likelihood ratio test= 26.01 on 1 df, p=3e-07
#> Wald test = 25.88 on 1 df, p=4e-07
#> Score (logrank) test = 29.33 on 1 df, p=6e-08
The simple Cox PH model with covariate gcs shows that with each one unit increase in gcs, the crude log hazard for death changes by a factor of \(-0.175\).
lets tidy it up
tidy(surv_data_gcs,
exponentiate = TRUE,
conf.int = TRUE)
#> # A tibble: 1 x 7
#> term estimate std.error statistic p.value conf.low conf.high
#> <chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
#> 1 gcs 0.840 0.0343 -5.09 0.000000363 0.785 0.898
next up we add surv_data type
surv_data_mv <-
coxph(Surv(time = time2,
event = status == 'dead') ~ stroke_type + gcs ,
data = surv_data)
tidy(surv_data_mv, exponentiate = TRUE, conf.int = TRUE)
#> # A tibble: 2 x 7
#> term estimate std.error statistic p.value conf.low conf.high
#> <chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
#> 1 stroke_typeIS 0.774 0.323 -0.794 0.427 0.411 1.46
#> 2 gcs 0.846 0.0357 -4.68 0.00000290 0.789 0.908
lets test if surv_data type is worth it
anova(surv_data_mv,surv_data_gcs,test="LRT")
#> Analysis of Deviance Table
#> Cox model: response is Surv(time = time2, event = status == "dead")
#> Model 1: ~ stroke_type + gcs
#> Model 2: ~ gcs
#> loglik Chisq Df Pr(>|Chi|)
#> 1 -187.48
#> 2 -187.80 0.6422 1 0.4229
- The variable is not significant
we try another one
surv_data_dm <-
coxph(Surv(time = time2,
event = status == 'dead') ~ dm + gcs ,
data = surv_data)
tidy(surv_data_dm, exponentiate = TRUE, conf.int = TRUE)
#> # A tibble: 2 x 7
#> term estimate std.error statistic p.value conf.low conf.high
#> <chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
#> 1 dmyes 0.625 0.328 -1.43 0.152 0.329 1.19
#> 2 gcs 0.840 0.0347 -5.04 0.000000466 0.785 0.899
lets test if dm is worth it
anova(surv_data_dm,surv_data_gcs,test="LRT")
#> Analysis of Deviance Table
#> Cox model: response is Surv(time = time2, event = status == "dead")
#> Model 1: ~ dm + gcs
#> Model 2: ~ gcs
#> loglik Chisq Df Pr(>|Chi|)
#> 1 -186.71
#> 2 -187.80 2.181 1 0.1397
- still not worth is it , we can go on and on…
lets do a backward elimination
fatal_mv1<-rms::cph(Surv(time2,status == 'dead') ~ gcs + stroke_type+dbp+wbc
+ sex + dm + sbp ,data = surv_data)
fastbw(fatal_mv1)
#>
#> Deleted Chi-Sq d.f. P Residual d.f. P AIC
#> wbc 0.07 1 0.7881 0.07 1 0.7881 -1.93
#> stroke_type 0.14 1 0.7130 0.21 2 0.9015 -3.79
#> sex 0.16 1 0.6934 0.36 3 0.9478 -5.64
#> sbp 0.39 1 0.5326 0.75 4 0.9447 -7.25
#> dbp 1.12 1 0.2897 1.87 5 0.8664 -8.13
#> dm 2.03 1 0.1542 3.90 6 0.6898 -8.10
#>
#> Approximate Estimates after Deleting Factors
#>
#> Coef S.E. Wald Z P
#> gcs -0.1762 0.03495 -5.041 4.635e-07
#>
#> Factors in Final Model
#>
#> [1] gcs
- final model has gcs variable
Validity of the Cox PH model
The Cox proportional hazards model makes several assumptions. We use residuals methods to:
- check the proportional hazards assumption with the Schoenfeld residuals
- detect nonlinearity in relationship between the log hazard and the covariates using Martingale residual
- examining influential observations (or outliers) with deviance residual (symmetric transformation of the martinguale residuals), to examine influential observations
Testing proportional hazard
The proportional hazard assumption is supported by a non-significant relationship between residuals and time, and refuted by a significant relationship.
We can test with the Goodness of Fit (GOF) approach based on the residuals defined by Schoenfeld.
The idea behind the statistical test is that if the PH assumption holds for a particular covariate then the Schoenfeld residuals for that covariate will not be related to survival time.
The implementation of the test can be thought of as a three-step process.
-
Step 1. Run a Cox PH model and obtain Schoenfeld residuals for each predictor.
-
Step 2. Create a variable that ranks the order of failures. The subject who has the first (earliest) event gets a value of 1, the next gets a value of 2, and so on.
-
Step 3. Test the correlation between the variables created in the first and second steps. The null hypothesis is that the correlation between the Schoenfeld residuals and ranked failure time is zero.
For each covariate, the function cox.zph() correlates the corresponding set of scaled Schoenfeld residuals with time, to test for independence between residuals and time. Additionally, it performs a global test for the model as a whole.
test.ph <- cox.zph(stroke_type)
print(test.ph)
#> chisq df p
#> gcs 0.0206 1 0.89
#> stroke_type 1.2745 1 0.26
#> dbp 1.0480 1 0.31
#> wbc 0.0235 1 0.88
#> sex 0.4560 1 0.50
#> dm 1.9324 1 0.16
#> sbp 1.2716 1 0.26
#> GLOBAL 6.1719 7 0.52
ggcoxzph(test.ph)

From the output above, the test is not statistically significant, and therefore the global test is also not statistically significant. Therefore, we can assume the proportional hazards.
In the graphical diagnostic using the function ggcoxzph() [in the survminer package], the solid line is a smoothing spline fit to the plot, with the dashed lines representing a +/- 2-standard-error band around the fit. From the graphical inspection, there is no pattern with time. The assumption of proportional hazards appears to be supported for the covariates sex (which is, recall, a two-level factor, accounting for the two bands in the graph).
another approach
Another approach is to graphically check the PH assumption by comparing -log–log survival curves. A log–log survival curve is simply a transformation of an estimated survival curve that results from taking the natural log of an estimated survival probability twice.
\(h(t, X) = h_{0}(t)e^{ \sum_{j=1}^{p} \beta_{j}X_{j}}\) which is equivalent to \(S(t,X) = [S_{0}(t)]^{e^{ \sum_{j=1}^{p} \beta_{j}X_{j}}}\)
Therefore, $$-ln(-ln(S(t, X)))$$
$$= -ln(-ln([S_{0}(t)]^{e^{ \sum_{j=1}^{p} \beta_{j}X_{j}}}))$$ $$= -ln[-ln(S_{0}(t))] - ln[e^{ \sum_{j=1}^{p} \beta_{j}X_{j}}]$$
$$= - \sum_{j=1}^{p} \beta_{j} X_{j} - ln[-ln(S_{0}(t))]$$
Therefore, the corresponding log–log curves for these individuals are given as shown here, where we have simply substituted \(X_1\) and \(X_2\) for \(X\) in the previous expression for the log–log curve for any individual \(X\).
$$ln(-ln(S(t, X_1))) - ln(-ln(S(t, X_2)))$$ $$= \sum_{j=1}^{p} \beta_{j} (X_{1j} - X_{2j})$$
The baseline survival function has dropped out, so that the difference in log–log curves involves an expression that does not involve time t. The above formula says that if we use a Cox PH model and we plot the estimated -log–log survival curves for two groups of individuals on the same graph, the two plots would be approximately parallel.
m = survfit(Surv(time2,status == 'dead') ~ stroke_type
,data = surv_data)
s = summary(m)
s_table = data.frame(s$strata, s$time, s$n.risk, s$n.event, s$n.censor, s$surv, s$lower, s$upper)
s_table = s_table %>%
rename(strata=s.strata, time=s.time, surv=s.surv, lower=s.lower, upper=s.upper) %>%
mutate(negloglogsurv=-log(-log(surv)))
plot<-ggplot(s_table, aes(x=time, y=negloglogsurv, color=strata)) +
geom_line(size=1.25) +
theme(text=element_text(size=16),
plot.title=element_text(hjust=0.5)) +
ggthemes::scale_colour_tableau()+
tvthemes::theme_avatar()+
ylab("-ln(-ln(S(t)))") +
ggtitle("-log-log plot of survival time by surv_data type")
plot

The two curve cross, therefore this result suggests that the two groups in surv_data type do not satisfy the PH assumption.
Testing influential observations
To test influential observations or outliers, we can visualize either: the dfbeta values or the deviance residuals.
-
- dfbeta values
This plot produces the estimated changes in the coefficients divided by their standard errors. The comparison of the magnitudes of the largest dfbeta values to the regression coefficients suggests that none of the observations is terribly influential individually.
ggcoxdiagnostics(surv_data_stype, type = "dfbeta",
linear.predictions = FALSE, ggtheme = theme_bw())

2) deviance residuals
The deviance residual is a normalized transformation of the martingale residual. These residuals should be roughly symmetrically distributed about zero with a standard deviation of 1.
-
Positive values correspond to individuals that “died too soon” compared to expected survival times.
-
Negative values correspond to individual that “lived too long”.
-
Very large or small values are outliers, which are poorly predicted by the model.
ggcoxdiagnostics(stroke_type, type = "deviance",
linear.predictions = FALSE, tvtheme = theme_avatar())

ggcoxdiagnostics(stroke_type, type = "martingale",
linear.predictions = FALSE, ggtheme = theme_bw())

ggcoxdiagnostics(stroke_type, type = "deviance", ox.scale = 'time')

ggcoxdiagnostics(stroke_type, type = "deviance", ox.scale = "linear.predictions")

ggcoxdiagnostics(stroke_type, type = "schoenfeld", ox.scale = "observation.id")
